In the intricate world of carbohydrate chemistry, the anomeric effect stands as a fundamental principle, governing the conformational preferences of substituents attached to the anomeric carbon, the carbon bearing the glycosidic linkage in carbohydrates. This effect, first recognized by W.N. Haworth, dictates that substituents at the anomeric carbon prefer to adopt an axial conformation, minimizing steric interactions with the bulky substituent on the neighbouring carbon.
However, this seemingly universal rule faces an intriguing exception known as the reverse anomeric effect (RAE), where certain substituents, particularly positively charged nitrogen substituents, defy the generalized anomeric effect and favour an equatorial conformation.
Anomeric Effect and Its Implications
Before delving into the RAE, it’s essential to grasp the concept of the anomeric effect. In carbohydrates, the anomeric carbon is a crucial point of linkage, connecting the sugar molecules to form intricate structures that play vital roles in biological processes. The anomeric effect, arising from the interplay of steric and electronic factors, dictates that substituents at the anomeric carbon prefer an axial conformation, minimizing steric clashes with the neighboring substituent. This preference has far-reaching implications for carbohydrate chemistry, influencing reactivity, stability, and biological recognition.
The Puzzling Exception: The Reverse Anomeric Effect
In the midst of this well-established principle of the anomeric effect, a puzzling exception emerged. Raymond Lemieux, a pioneer in carbohydrate chemistry, observed in the 1960s that certain positively charged nitrogen substituents attached to the anomeric carbon exhibited a preference for an equatorial conformation, contradicting the predictions of the generalized anomeric effect. This unexpected observation gave rise to the concept of the reverse anomeric effect (RAE), a phenomenon that challenged conventional wisdom and sparked intense debate among chemists.
Theories Behind the Reverse Anomeric Effect
Intrigued by this anomaly, scientists sought to unravel the underlying reasons behind the RAE. Two primary explanations emerged. The first, known as the electrostatic explanation, attributes the equatorial preference to stabilizing interactions between the lone pair electrons on the anomeric oxygen and the positively charged nitrogen substituent. These electrostatic interactions, akin to a dipole-dipole attraction, favour an equatorial arrangement, where the lone pairs and the positive charge are positioned in close proximity.
An alternative explanation, termed the delocalization model, suggests that the RAE arises from delocalization of the sp3 electrons of the anomeric carbon and the oxygen lone pair. In this scenario, the positively charged nitrogen substituent stabilizes the delocalized orbital, leading to a preference for an equatorial conformation.
Experimental Evidence Supporting the Reverse Anomeric Effect
Despite the ongoing debate surrounding the existence and generality of the RAE, compelling experimental evidence supports its occurrence in various molecules. One notable example is N-pyranosylimidazolides, where the positively charged imidazolium substituent at the anomeric carbon exhibits a strong preference for an equatorial conformation. Similarly, quaternary ammonium salts of carbohydrates also exhibit the RAE, further strengthening the evidence for this intriguing phenomenon.
Debates and Uncertainties: Questioning the Generality of the RAE
While the RAE has gained recognition in carbohydrate chemistry, it remains a subject of debate and ongoing research. Some scientists question the generality of the RAE, arguing that it might be restricted to specific substituents and conformations. Others propose alternative interpretations for the observed equatorial preferences, suggesting that factors beyond the anomeric effect might be in play.
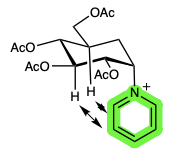
Implications and Applications: Expanding the Realm of Carbohydrate Chemistry
The RAE, despite its enigmatic nature, has significant implications for our understanding of carbohydrate chemistry and its potential applications. It challenges the generalized anomeric effect, expanding our knowledge of conformational preferences in carbohydrates. Additionally, the RAE holds potential in areas such as carbohydrate synthesis and drug design, providing new insights into manipulating carbohydrate structures for specific biological effects.
The existence of a reverse anomeric effect would have enormous consequences in terms of chemical reactivity of reactions that involve formation of a positively charged group at C-1 at some point during the transformation.
It might be expected that the hydrolysis of alpha-glycoside, would involve a ring flip, whereas the beta-glycoside would not. Explained in following figure:
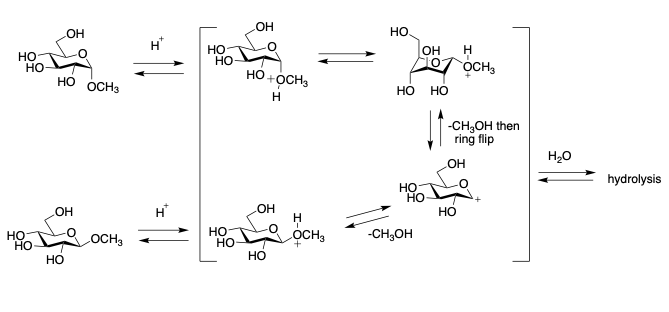
Conclusion: A Beacon for Further Exploration
The reverse anomeric effect stands as a fascinating anomaly in carbohydrate chemistry, challenging established principles and opening new avenues for exploration. While its exact nature remains a subject of debate, the RAE serves as a beacon for further research, encouraging scientists to delve deeper into the intricate world of carbohydrate conformations and their far-reaching implications. As we continue to unravel the mysteries of the RAE, we gain a deeper appreciation for the complexities and nuances of carbohydrate chemistry, expanding our understanding of these molecules that play essential roles in life’s processes.


