In the intricate domain of carbohydrate chemistry, Fischer glycosylation stands as a cornerstone reaction, a testament to the ingenuity of Emil Fischer, the Nobel laureate who first elucidated its mechanisms in the late 19th century. This article delves into the fascinating world of Fischer glycosylation, decodes its intricacies and exploring its enduring significance in modern synthetic chemistry.
Embarking on the Fischer Glycosylation Odyssey
Fischer glycosylation, quite simply, is the art of forging a glycosidic linkage, the covalent bond that unites a carbohydrate, or sugar, to another molecule, often an alcohol. This reaction, orchestrated by an acidic catalyst, transforms the sugar into a glycoside, a versatile building block with far-reaching applications in the synthesis of complex carbohydrates.
The Fischer glycosylation saga unfolds in an acidic arena, typically employing strong acids like sulfuric or hydrochloric acid. These acidic maestros protonate the sugar’s anomeric hydroxyl group, rendering it susceptible to attack by the alcohol, the eager recipient of the sugar’s glycosyl moiety.
Navigating the Labyrinth of Products
As the Fischer glycosylation drama unfolds, a symphony of products emerges, each with its unique structural identity. The reaction can yield a mixture of ring sizes, pyranoses and furanoses, depending on the sugar’s conformational preferences. Additionally, the anomeric configuration, the orientation of the anomeric hydroxyl group, can be either alpha or beta, leading to a diastereomeric pair of glycosides.
Complex process, detailed mechanism not definitely known, despite much investigation. (Reviewed: B. Capon, Chem. Rev., 1969, 69, 407-498).
Generally agreed upon mechanistic feature:
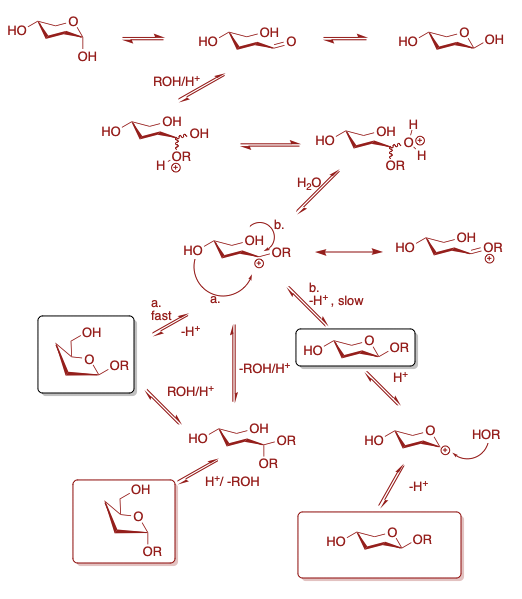
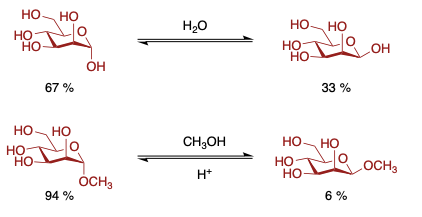
Equilibrim mixture of methyl glycosides usually show greater concentration of alpha-pyranoside than the reducing sugar. Results from a greater anomeric effects which is usually atributed to the solvent. Methanol is less polar than water and thus the anomeric effect is greater.
Harnessing the Power of Fischer Glycosylation
Despite its inherent complexity, Fischer glycosylation remains a cornerstone of synthetic carbohydrate chemistry, offering a robust and versatile platform for constructing intricate carbohydrate structures. Its applications span a wide spectrum, from the synthesis of natural products to the development of therapeutic agents and functional materials.
In the realm of natural product synthesis, Fischer glycosylation has enabled the synthesis of complex carbohydrates found in nature, such as those adorning the surfaces of cells or embedded within intricate biomolecules. These synthetic endeavors provide valuable insights into the structure and function of these naturally occurring carbohydrates.
Therapeutic Triumphs and Functional Forays
The therapeutic realm has also witnessed the transformative power of Fischer glycosylation. The synthesis of glycoconjugate vaccines, where a carbohydrate is conjugated to a protein or carrier molecule, has revolutionized vaccine development, offering enhanced protection against a myriad of diseases.
Beyond therapeutics, Fischer glycosylation has paved the way for the development of functional materials with remarkable properties. The synthesis of glycoconjugate polymers, where carbohydrates are tethered to polymer backbones, has led to the creation of materials with enhanced water solubility, biocompatibility, and selective binding capabilities.
Conclusion: A Legacy of Innovation
Fischer glycosylation, a testament to the brilliance of Emil Fischer, stands as a cornerstone of synthetic carbohydrate chemistry. Its ability to forge glycosidic linkages, the foundation of carbohydrate chemistry, has revolutionized the synthesis of complex carbohydrates, opening doors to diverse applications in natural product synthesis, therapeutics, and functional materials. As we continue to explore the intricate world of carbohydrates, Fischer glycosylation will undoubtedly remain an indispensable tool, shaping the future of carbohydrate chemistry.
