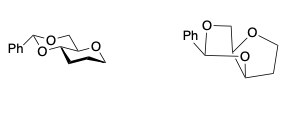
In the intricate world of organic chemistry, carbohydrates stand as fundamental building blocks, playing pivotal roles in various biological processes. To safeguard these crucial molecules during synthetic transformations, chemists employ ingenious strategies, one of which involves the formation of acetals between diols.
Acetals: The Unsung Heroes of Protection:
Acetals – versatile compounds that act as the guardians of carbohydrates during synthetic transformations. The formation of acetals involves the reaction between a carbonyl group, typically an aldehyde or ketone, and a diol. This process results in the creation of a stable acetal, effectively shielding the carbonyl group from unwanted reactions.
In some cases possible to form acyclic or “mixed” acetals, but in general cyclic acetals are of more use.

Acid Catalysts Frequently Used:
- p-TsOH, camphorsulfonic Acid, H2SO4, HCO2H, HCl
- Resin bound H+ (Dowex etc)
- ZnCl2, CuSO4, FeCl3
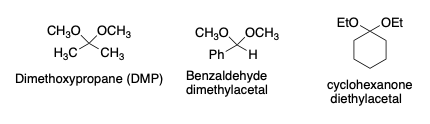
Typical Carbonyls
- Benzaldehyde, acetone, substituted benzaldehydes, cyclohexane, cyclopentanone, acetaldehyde, formaldehyde
- Carbonyl equivalents – Acetals
Carbonyl equivalents – enrols ethers
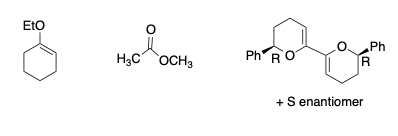
Product formation is heavily dependent upon the stereochemistry of the sugar involved.
Most frequent cyclic structures involve the formation of dioxolane rings between 1,2 diols or 1,3-dioxane rings between 1,3 diols.
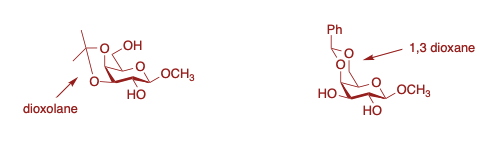
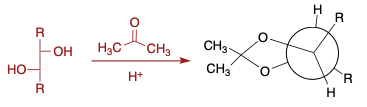
Acetal Formation on Acyclic Diols (Alditols)
General comments about dioxolane formation
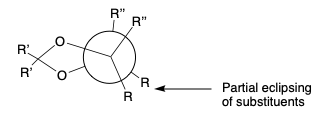
- erythro- diols from dioxolanes that have eclipsed R groups. Formation is slow.

- thero- diols from dioxolanes in which R groups are not eclipsed. Formation is fast.
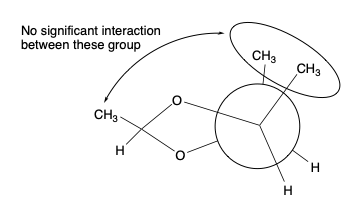
- Dioxolanes from aldehydes and erythro– diols. More stable form has the alkyl group on the same side as the other substituents.

General comments about 1,3-dioxane formation.
- C-O bond shorter (1.43 Å) than the C-C bond (1.54 Å) and hence the dioxanes are not perfect chairs.
- Due to these bond lengths, 1,3 diaxial interactions involving a group at C-2 are larger in 1,3 dioxins than in cyclohexanes.
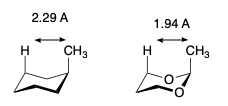
- Results in a significant shift in the equilibrium between conformers.

- Reaction of 1,3 diols with ketones. Show, because you always have an axial group at C-2.
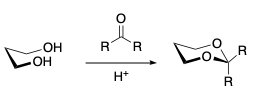
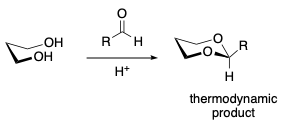
- Reactions of 1,3 diols with aldehydes. Fast, thermodynamic product with the equatorially substituted alkyl group is formed.
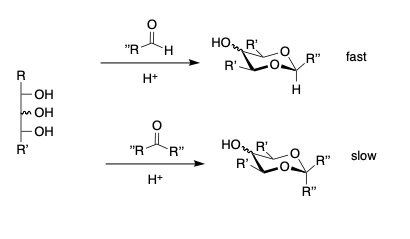
- Reaction of β-erythro-diols. Aldehydes react faster than ketones.
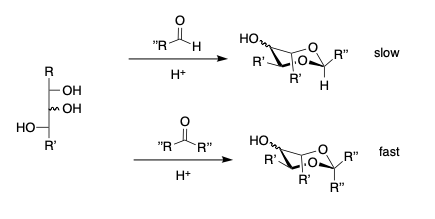
- Reaction of β-threo-diols. Aldehydes react slowly and ketones essentially not at all.
Summary:
Reactions of alditols with ketones
- Gives dioxolanes (avoids unfavourable 1,3 diaxial interactions in dioxanes).
- α-threo diols react faster than α-erythro diols.
Reactions of alditols with aldehydes.
- Gives predominantly dioxanes (eclipsing of substituents in dioxolanes makes the dioxanes more stable).
- β-erthyro diols react faster than β-threo diols.
- Thermodynamic product (equatorial C-2 substituent) predominates.
Acetal Formation on Cyclic Sugars (Reducing Sugars)
- Product Formation depends on both the stereochemistry of the OHs involved as well as the stability of the carbohydrate ring.
General trends
- Dioxolane formation on trans-1,2 diols, under normal conditions, does not occur. Can’t form trans-fused-5-membered rings.
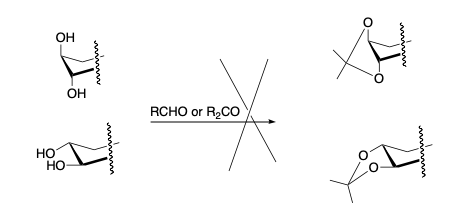
- Dioxolane Formation on cis-1,2 diols will form, but slowly.
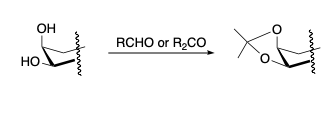
- Ring structure makes it impossible for the most stable dioxolane (from α-threo-diols) to form therefore the α-erythro-reacts to give the product.
- Fusion of the dioxolane ring onto the chair causes it to flatten. Flattening of the chair sometimes brings trans-diols close enough together to react.
- (-) Inositols
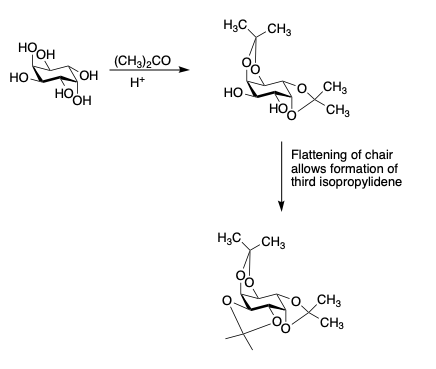
- Dioxane formation occurs most readily between o4 and o6 on pyranosides and between o5 and o3 on furanosides.