Cyclic Acetal Formation on Reducing Sugars
Frequently results in the production of a number of products via the formation of acetals on both the furanose and pyranose isomers.
– Leading references
- Gelas, J. Adv. Carbohydrate. Chem. Biochem., 1981, 39, 71-156.
- de Belder, A. N. Adv. Carbohydr. Chem. Biochem., 1977, 34, 179-241.
- de Belder, A. N. Adv. Carbohydr. Chem. Biochem., 1965, 20, 219-302.
- Brady Jr., R. F. Adv. Carbohydr. Chem. Biochem., 1971, 26, 197-278.
- Close, D. M. Chem. Rev., 1979, 79, 491 -513.
Most useful derivatives are isopropylidene acetals.
- In general the sugar will add as many equivalents of acetone as possible; dictated by relative stereochemistry of the hydroxyl groups on the sugar.
- Formation typically involves the suspension or dissolution of the sugar in acetone or dimethoxypropane followed by the addition of the acid. Solution becomes homogenous as it reacts. This method leads to the thermodynamic products.
- Kinetic products can be obtained by using a cosolvent (DMF, CH3CN) and 1-4 equivalents of either dimethoxypropane or 2-methoxypropane or 2-methoxypropene as the isopropylidenation reagent.
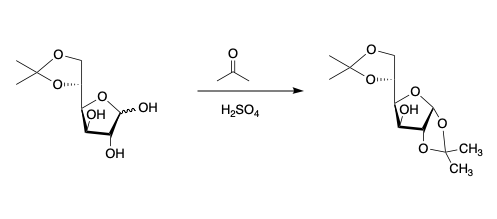
Formation of thermodynamic isopropylidene products with glucose, mannose and galactose.
Glucose
- Methods in Carbohydrate Chemistry, Volume 2, page 318.
- Pyranose forms of the sugar predominate at equilibrium and the kinetic product is the 4,6-isopropylidene.

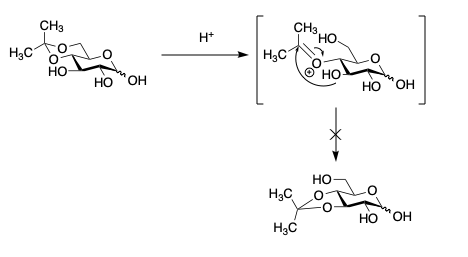
- Due to axial CH3 in the dioxane, rearrangement is favoured; however , all 2° OH groups on the pyranose from are trans and so acetal migration via the intermediate below does not occur.
- Instead, the ring isomerizes to the furanose form as below giving the 5,6-isopropylidene.
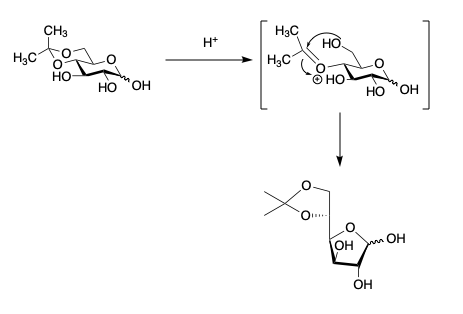
- Anomerization and then acetonation gives the product.